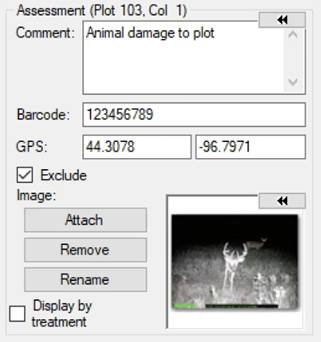
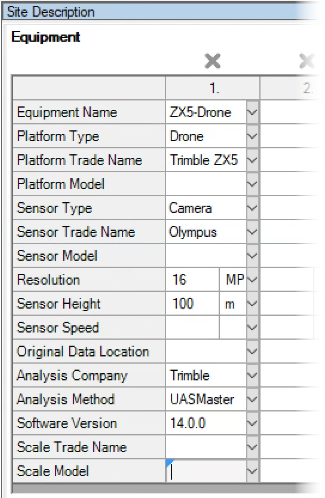
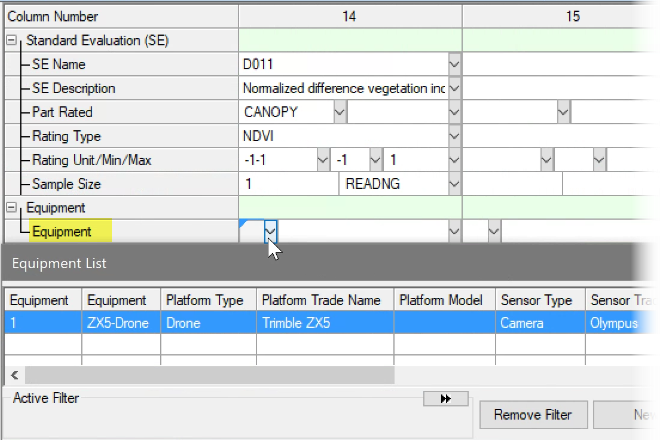
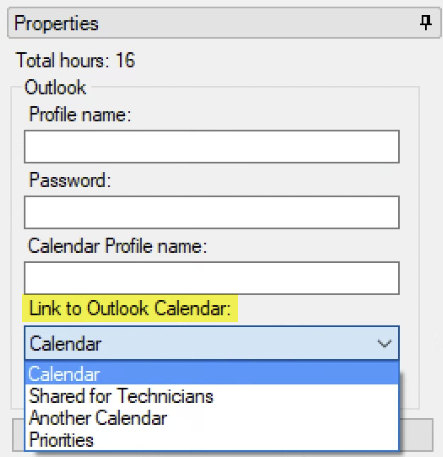
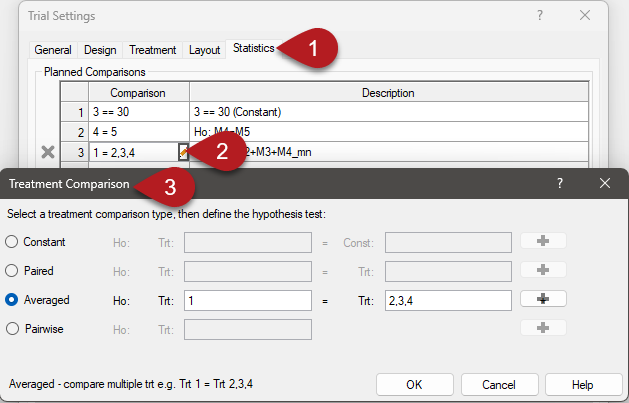
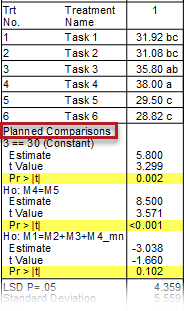
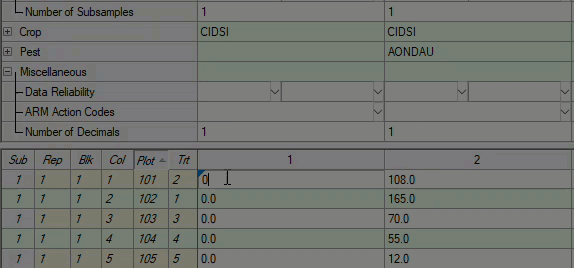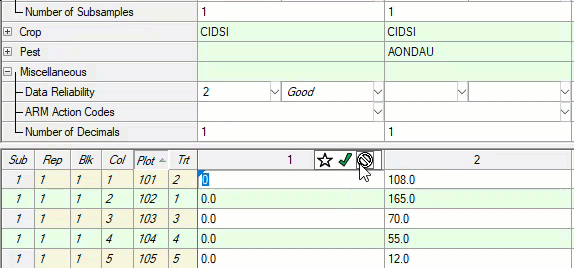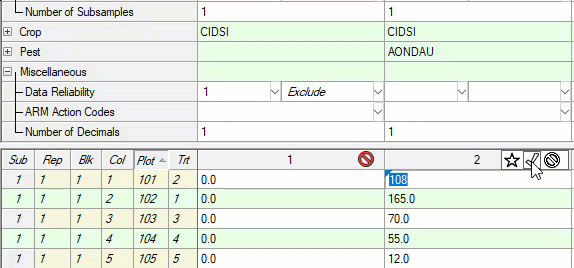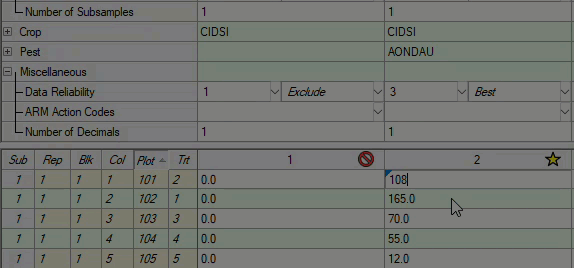
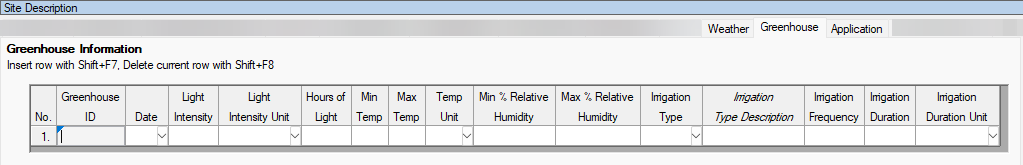
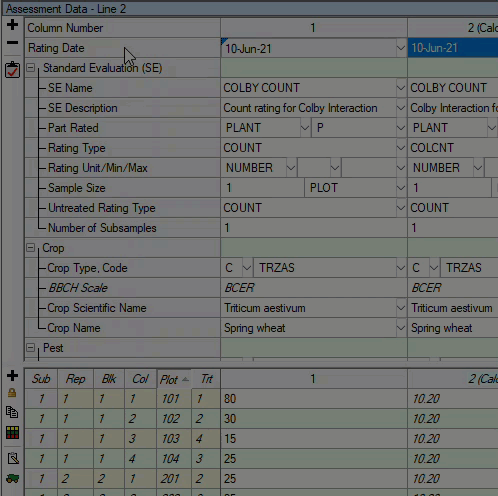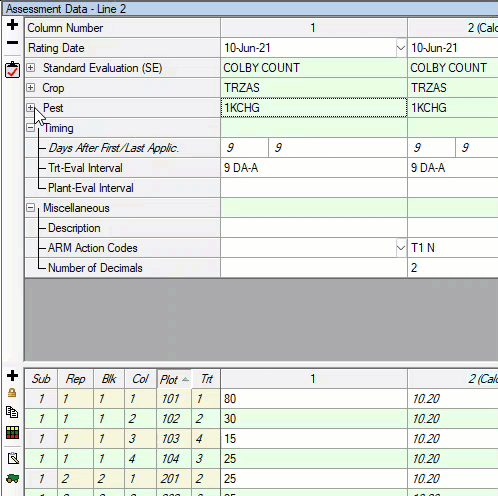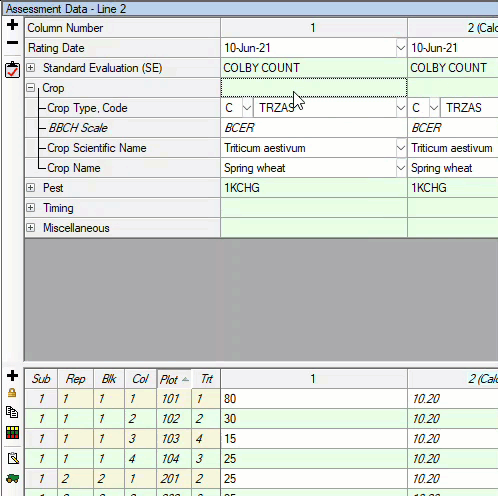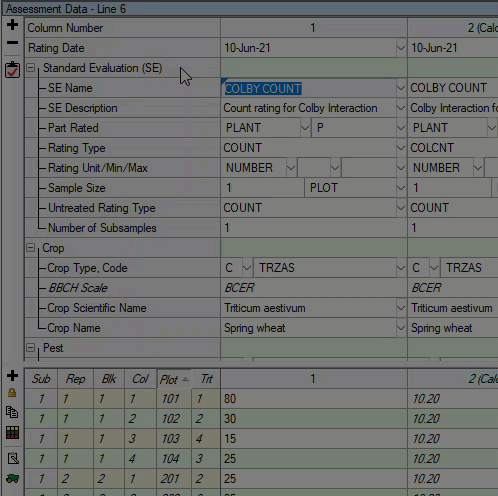
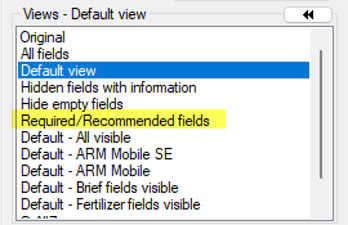
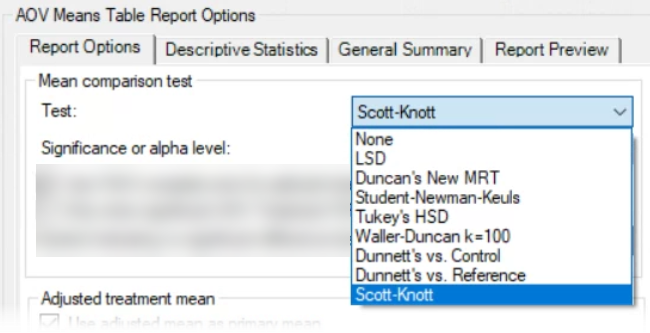
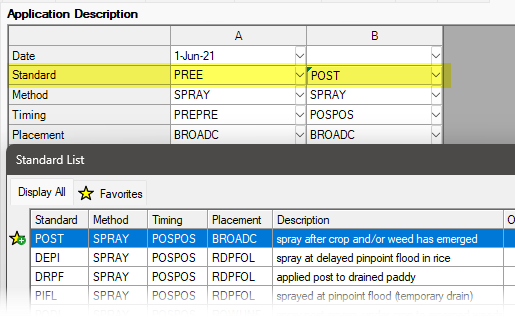
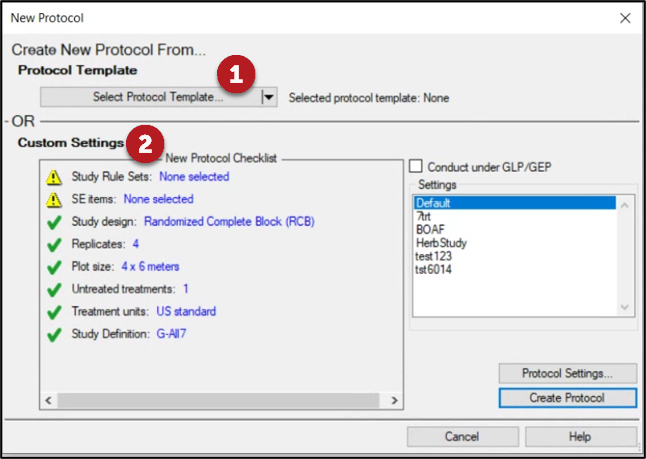
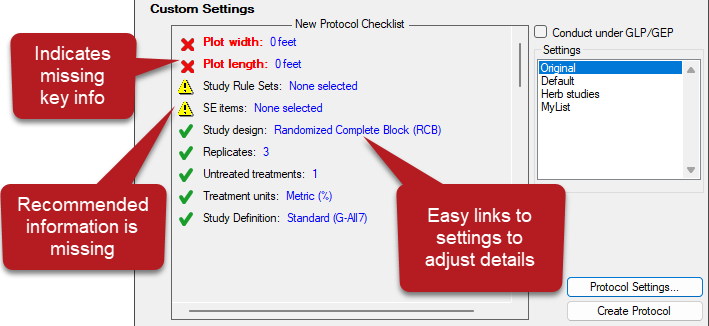
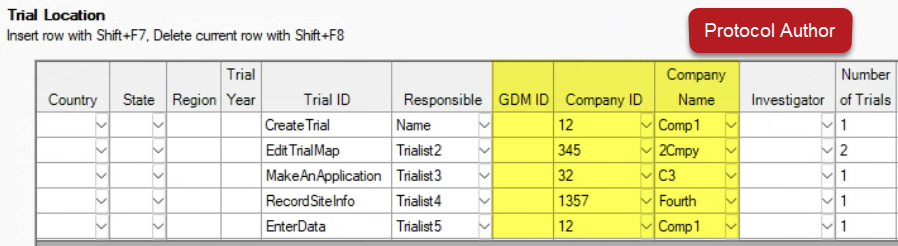
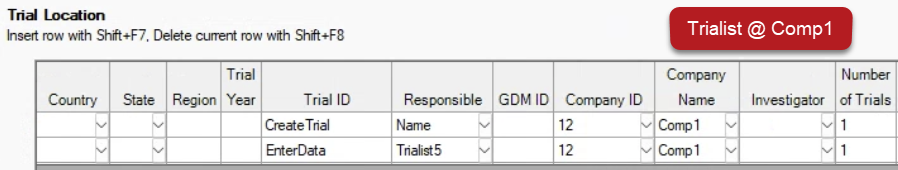
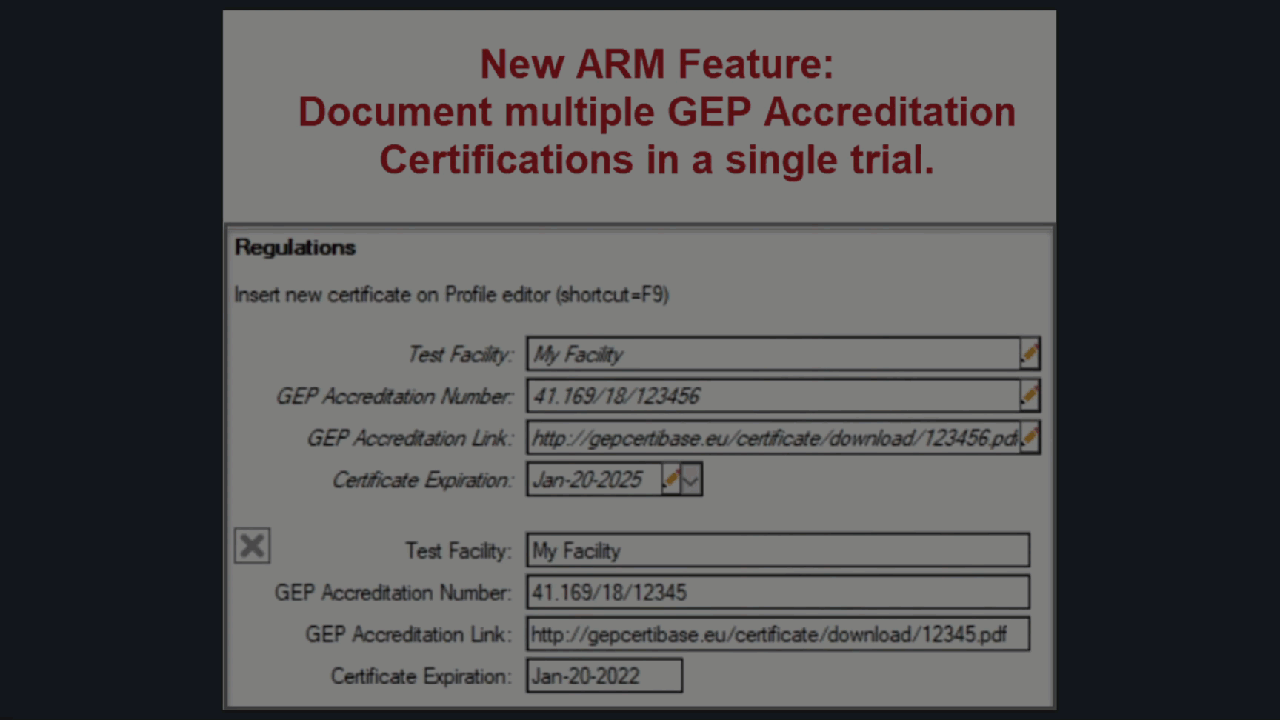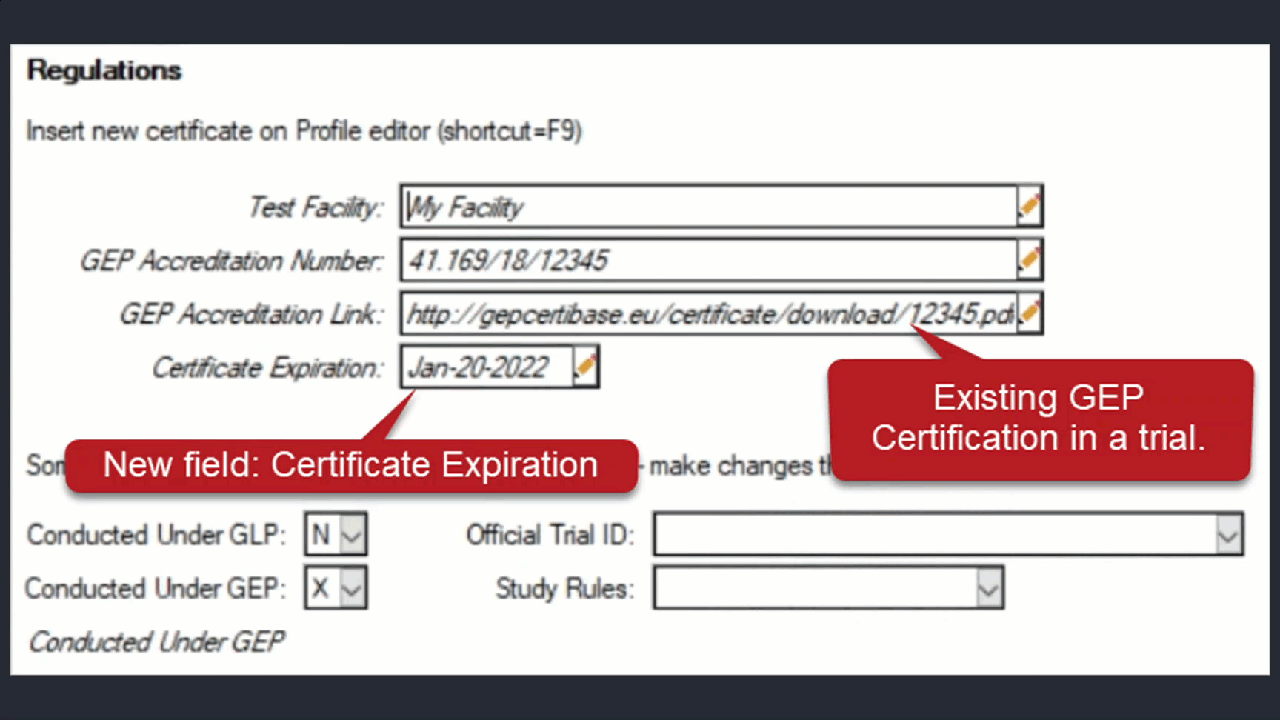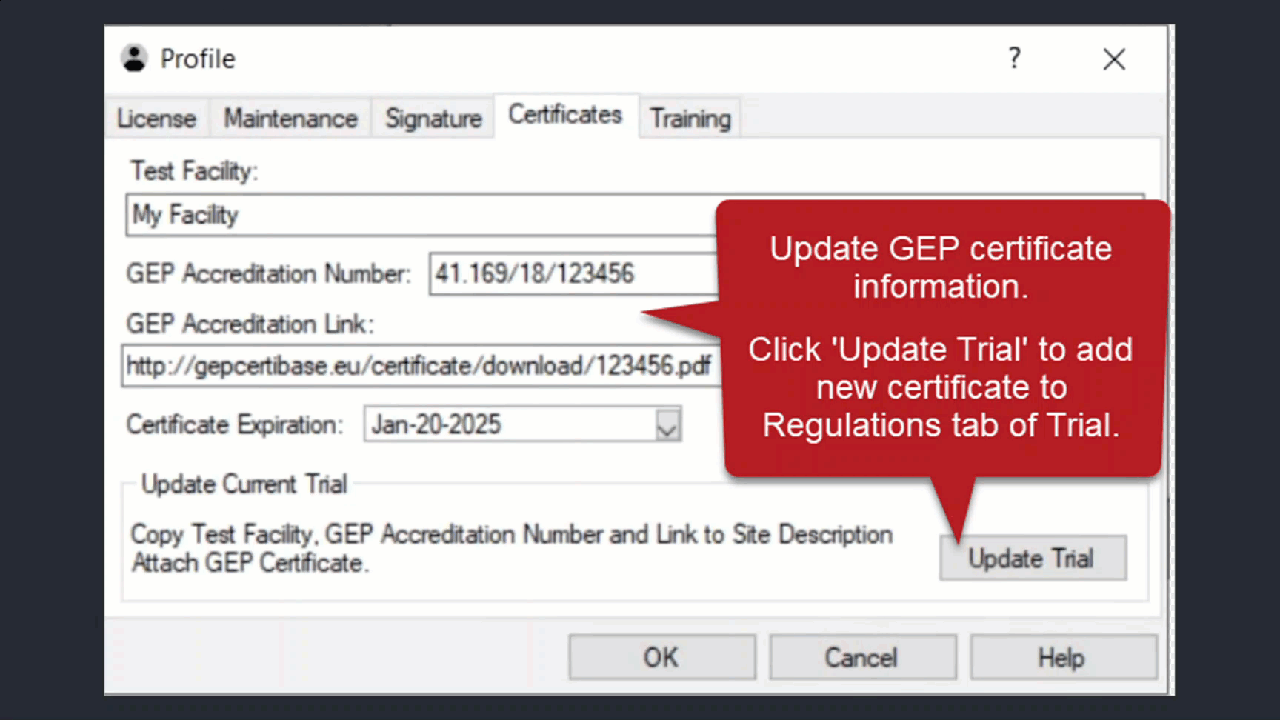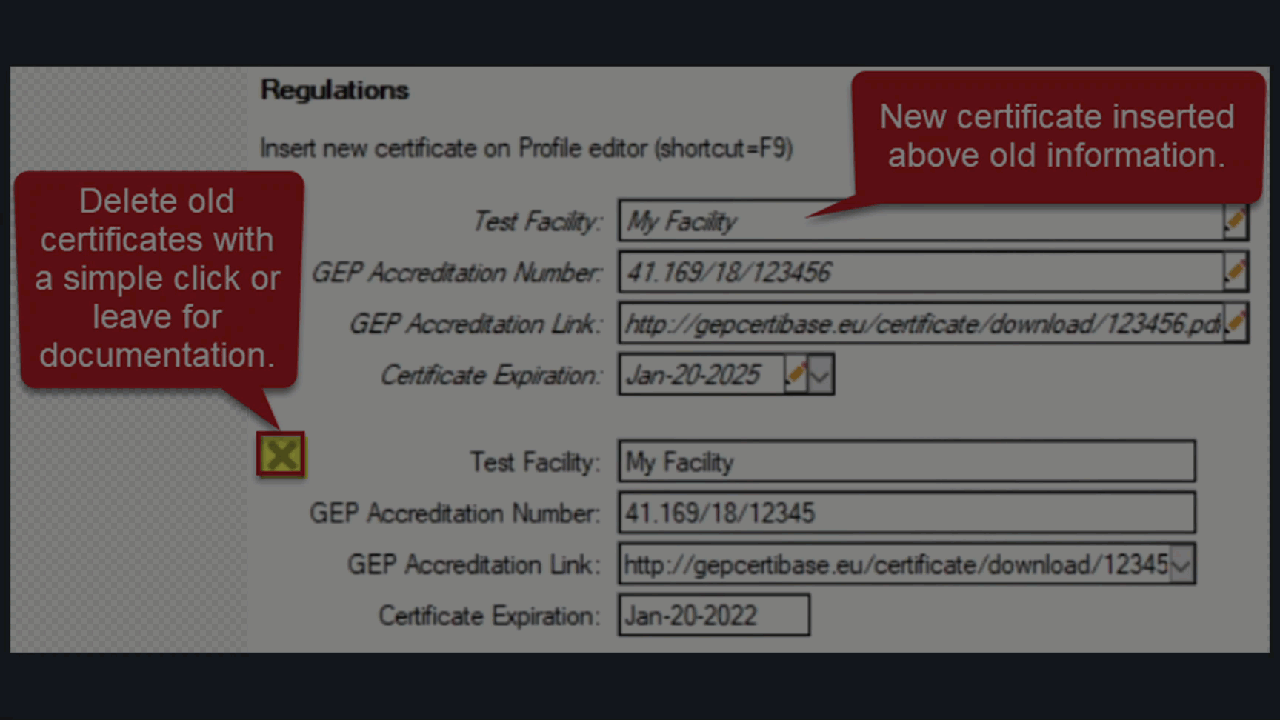
See ARM 2023.x Features (pdf) for a presentation of features added to ARM this year.
- Watch the PowerPoint version in presentation mode to view embedded feature-in-action videos.
See the Change Log (txt) for a complete list of all changes made in this (and previous) versions of ARM.