Set up a complete protocol with these more advanced features and tips about ARM protocols.
Introduction to Study Rules (7:34)This video introduces Study Rules and how to leverage them to improve the consistency of trial data and protect critical information.
|
||
 |
Advanced Study Rules (11:37)This video takes a deeper dive into ARM STudy Rules. We will review GDM's recommended Study Rules and how to leverage them to improve your research. Watch our Intro to Study Rules tutorial video for a basic overview of study rules.
|
|
Basics of Standard Evaluations (SEs) (6:28)This video will demonstrate how to utilize Standard Evaluations to save time and improve data consistency. A Standard Evaluation, or SE, is a standardized rating to be performed. The user creates a file that saves the header description for an assessment.
|
||
 |
Creating SEs (5:38)Modify existing SEs from the Master List or create your own custom SE.
|
|
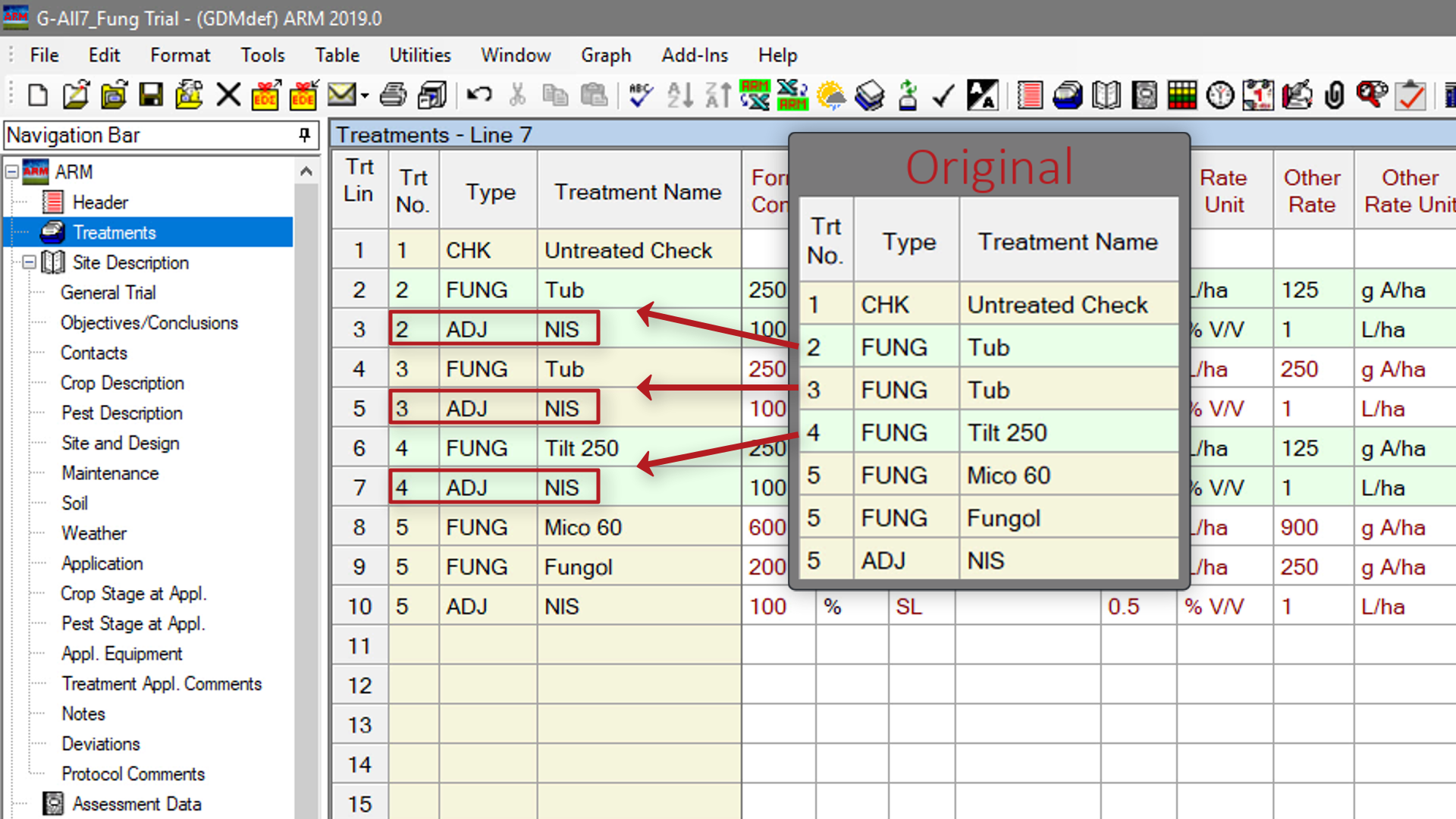
Duplicate Treatment Lines with Paste Special (1:30)Use Paste Special to quickly duplicate treatment lines, for example adding an adjuvant to many treatments at the same time.
|
||
Creating a Split-Plot Factorial Study (8:21)Learn how to set up a Split-Plot (factorial) protocol in ARM, and how the trial will be randomized.
|
||
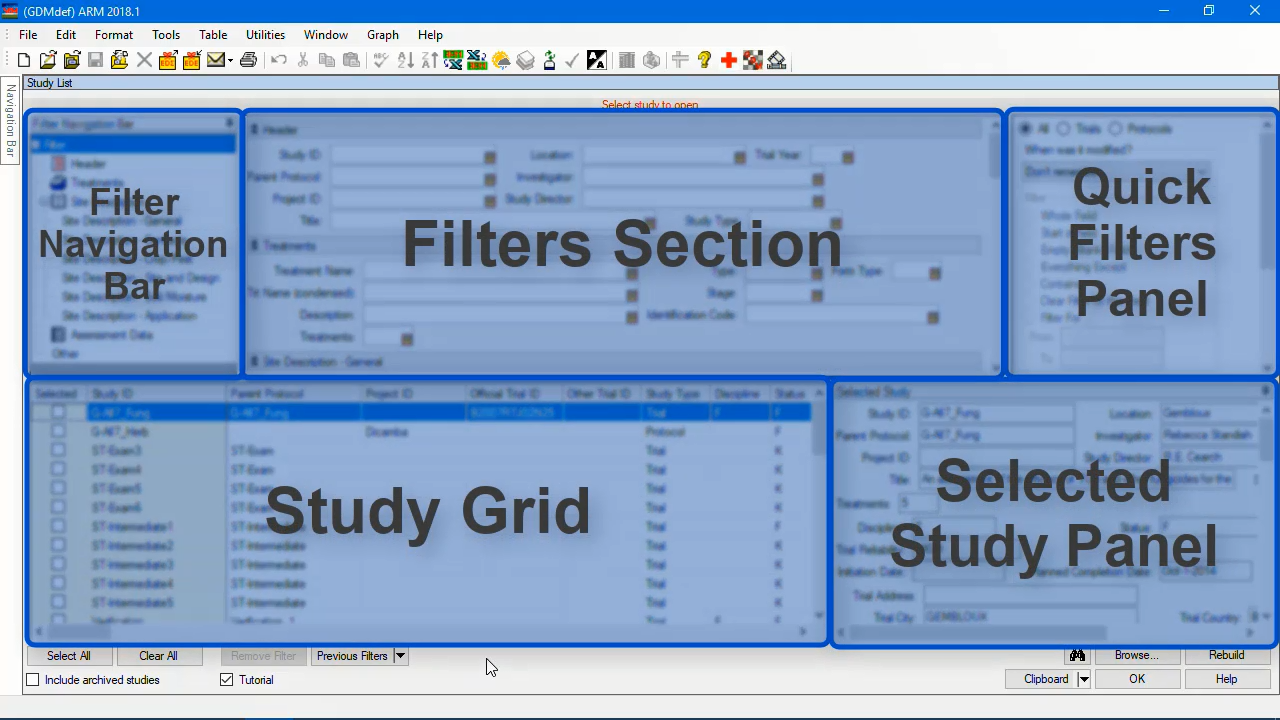
Search across ARM files with the Study List (4:17)Learn the basics of using the study list tool in ARM.
|
||
 |
Trial Location Table (2:54)Learn how to plan and organize trial locations in ARM using the Trial Location Table.
|
|
| < Back to video tutorials |